Improvement in all domains of the female sexual function index (FSFI)1*
At 12 Weeks, Survey Results Demonstrated an Improvement in Total Score
of 62% with INTRAROSA vs Baseline1†
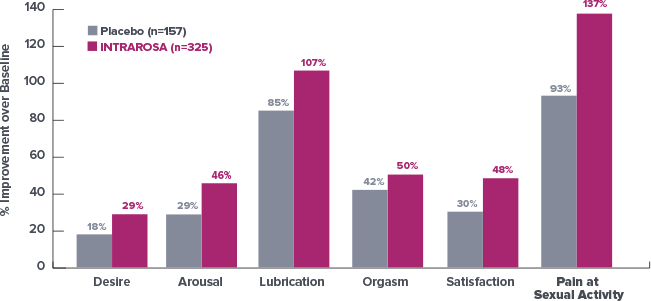
- The study was
performed in a
population of
women with VVA
having moderate
to severe dyspareunia
as the most bothersome
symptom but not in a
population enrolled as
having the diagnosis of
hypoactive sexual desire
disorder (HSDD) with distress due to HSDD. Further clinical studies are required to determine safety and efficacy of INTRAROSA for treatment of
sexual dysfunction
At 12 Weeks, Survey Results Demonstrated an Improvement in Total Score of 62% with INTRAROSA vs Baseline1

- Limitations: This post-hoc analysis of Study 2 data is not powered to show statistical significance. The study population did not have a diagnosis of sexual dysfunction. Further clinical studies are required to determine safety and efficacy of INTRAROSA for treatment of sexual dysfunction
Indication
INTRAROSA is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
Important Safety Information
INTRAROSA is contraindicated in women with undiagnosed abnormal genital bleeding.
Estrogen is a metabolite of prasterone. Use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer. INTRAROSA has not been studied in women with a history of breast cancer.
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥2 percent was vaginal discharge. In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥2 percent were vaginal discharge and abnormal Pap smear.
To report SUSPECTED ADVERSE REACTIONS, contact Millicent Pharma at 1-877-810-2101 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
References: 1. Labrie F, Derogatis L, Archer DF, et al. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401‐2412. 2. Intrarosa [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2020.

