Demonstrated safety profile
Safety was demonstrated in four 12-week placebo-controlled trials
and one 52-week open-label trial1
Adverse reactions with an incidence ≥2%
Abnormal Pap smear
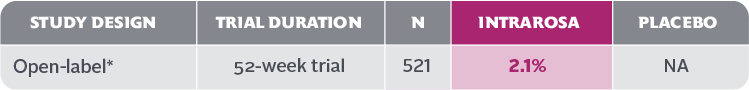
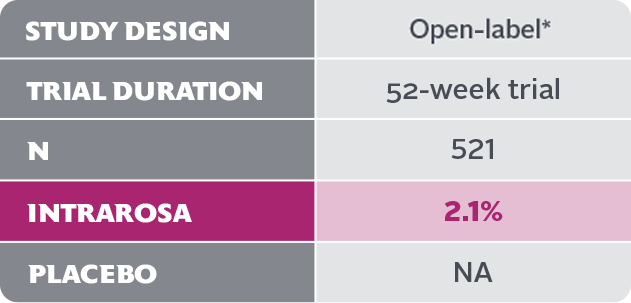
- In the 52-week INTRAROSA clinical trial, 11 cases of abnormal Pap smear included 1 case of low-grade squamous intraepithelial lesion (LSIL) and 10 cases of atypical squamous cells of undetermined significance (ASCUS)
No FDA boxed warning1
Vaginal discharge
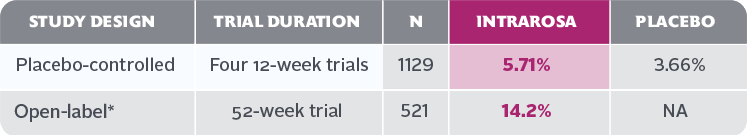
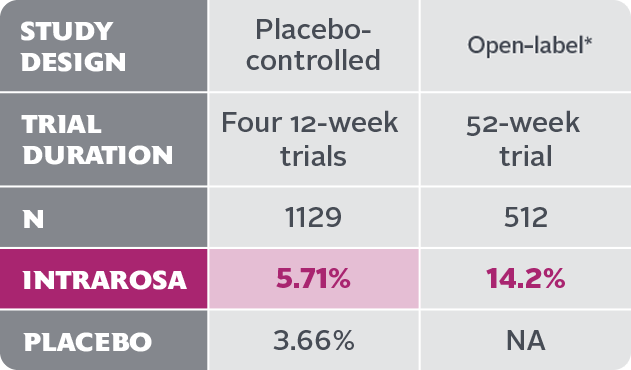
- Vaginal discharge is the most frequently reported treatment-emergent adverse reaction in the INTRAROSA treatment group, with an incidence of ≥2% vs the placebo group
No FDA boxed warning1
Indication
INTRAROSA is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
Important Safety Information
INTRAROSA is contraindicated in women with undiagnosed abnormal genital bleeding.
Estrogen is a metabolite of prasterone. Use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer. INTRAROSA has not been studied in women with a history of breast cancer.
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥2 percent was vaginal discharge. In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥2 percent were vaginal discharge and abnormal Pap smear.
To report SUSPECTED ADVERSE REACTIONS, contact Millicent Pharma at 1-877-810-2101 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
Reference: 1. Intrarosa [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2020.

